
GRADUATE RESEARCH DAY SHOWCASE & COMPETITION
On Graduate Research Day student researchers get the stage. It is a day long opportunity for graduate students to gain valuable professional development experience, network with industry partners, present research, and learn about the research of others.
SPRING 2021 PROJECTS
- 01 Peeyush Awasthi
- 02 Tommaso Benigni
- 03 Tananant Boonya-ananta
- 04 Kacie Kaile
- 05 Steafan Khan
- 06 Kevin Leiva
- 07 Manuel Perez
- 08 Edwin A. Robledo
- 09 Lin Tong
Student Name: Peeyush Awasthi
Title: jaBCI – Validation of an equivalent noninvasive model of iBCI
co-authors: Hsiang Lin
Advisor: Zachary Danziger
Lab Link: https://anil.fiu.edu/
Abstract: Among all iBCI (intracortical brain computer interface) based BCIs are used to capture the intention as firing rates of neurons located in any specific cortical segment like arm area of motor cortex to translate them to control an end effector like robotic arm or cursor etc. Although iBCI is credited with the highest spatial and temporal resolution together but suffers with the critical problem of long-term sustainable recordings of single unit activity or multi-unit activity or LFP. It is an invasive method and also suffers with statistical low power because of limited number of human participant or non-human primates (NHP) as subjects in any of the studies. The jaBCI (joint angle Brain computer interface) presents a machine learning based, biomimetic model for an iBCI with a large number of participants comparing results and cursor control aligned to other iBCI study based on humans. Our study also investigates a trained neural network dependent approach to effectively bio-mimic the cortical activity and also preserves the variability of the neural activity across sessions recorded on subjects used to train the neural network based iBCI emulator model. Emulated activity passes through multilayered evaluation metric for both offline and online method. The jaBCI model evolves as a noninvasive economic alternative eliminating the low statistical power of iBCI based BCIs. Human participants use their fingers to provide an input with high degree of freedom to generate emulated cortical activity to control end effector through a decoder as applied for actual iBCI.
Student Name: Tommaso Benigni
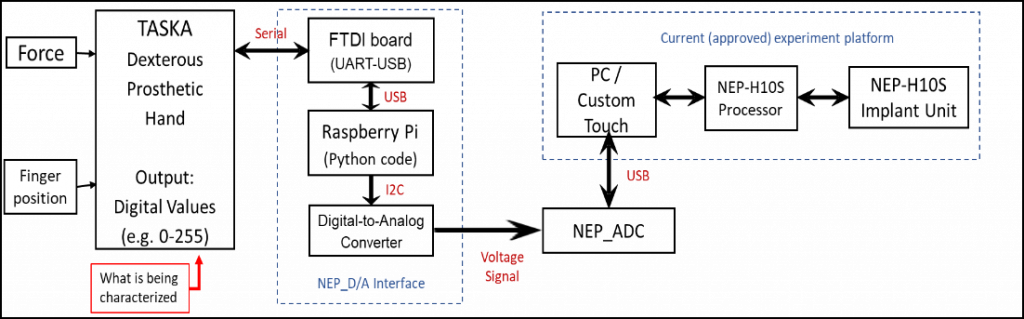
Title: Characterization and Integration of a Sensorized Dexterous Prosthetic Hand to Increase Simultaneous Perceivable Sensations for a Neural Enabled Prosthetic Hand.
co-authors: Sathyakumar Kuntaegowdanahalli, Andres Pena, James Abbas, Ranu Jung
Advisor: Ranu Jung
Lab Link: https://ans.fiu.edu
Abstract: On-going clinical trials of a Neural-Enabled Prosthetic Hand system in individuals with upper-limb amputation, attempt to elicit sensory percepts through electrical stimulation of nerve fibers in peripheral nerves of the residual limb. Currently, a single force and position sensor in a one degree of freedom instrumented prosthetic hand are being used to drive the electrical stimulation. Due to this limited number of sensor inputs, only two sensory percepts can be simultaneously stimulated. By increasing sensor inputs, we allow for new experiments assessing the ability of users to combine multiple sensory percepts in both an in-lab setting and at home. To accomplish this a commercially available sensorized dexterous prosthetic hand the TASKATM was selected to be integrated with the NEP system. The TASKATM contains a force sensitive resistor at the tip of each finger and a motor encoder at each base which output digital values ranging from 0-255. All twelve of these sensors were characterized to ensure they met the requirements for integration with the rest of the system. Using a calibrated force sensor and a custom-made motion tracking software, each individual sensor was characterized. Results show the FSRs have an average resolution of <.3 N/FSR value, while the Encoders had an average of resolution of <.6o/ value. Most sensors met the requirements with only the thumb FSR presenting a software error. With the characterization complete an interface was developed to use the TASKATM in-lab to provide feedback from multiple sensor inputs into a single percept to a participant. Future work includes development of and FDA approval of an interface for participants to use the TASKATM with the NEP at Home.
Student Name: Tananant Boonya-ananta
Title: Synthetic Photoplethysmographic Waveform at the Radial Artery
co-authors: Andres J. Rodriguez, Ajmal, Vinh Nguyen Du Le, Joshua D. Hutcheson, Jessica C. Ramella-Roman
Advisor: Dr. Jessica C. Ramella-Roman
Lab Link: https://web.eng.fiu.edu/jramella/
Abstract: Obesity is a significant risk factor for development and management of cardiovascular disease, one of the leading 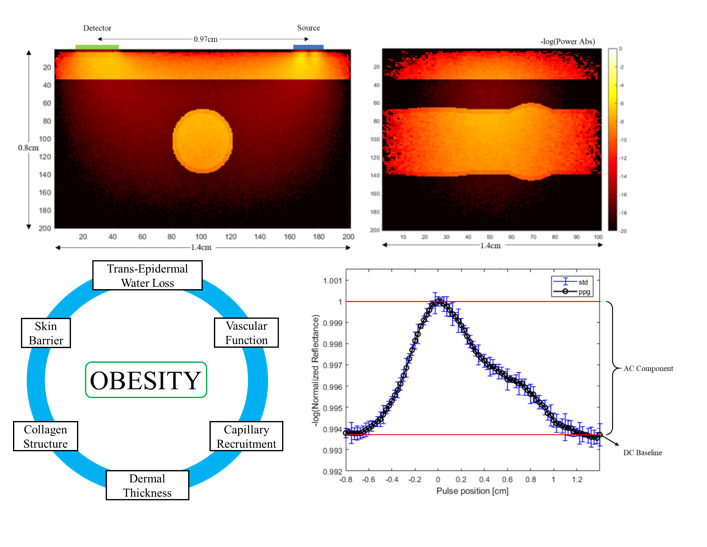 causes of death in the United States. Blood pressure (BP) is a key factor for monitoring cardiac health. Development of new optical wearable devices with capabilities to accurately measure blood pressure is becoming more prevalent and desirable. We have developed a model to generate synthetic photoplethysmographic waveforms captured by a commercial device for the radial artery at the volar surface of the wrist. This model incorporates the combination of Finite Element Model used to represent geometric changes to the radial artery and Monte Carlo light transport model to assess the interaction of optical signal with the physiological media. We highlight the effects of increasing BMI and changes attributed to obesity and epidermal melanin content on the collected PPG signal. Changes associated with obesity include trans-epidermal water loss, dermal blood content, dermal thickening, and arterial depth. We analyze the separation of PPG signal from different layers of vasculature to differentiation the signal components from the dermal plexus and main artery pulsation under 130 to 150mmHg blood pressure. Recognition of factors which effects the PPG signal is critical in accurate representation of cardiac health. Understanding changes to the PPG waveform morphology allows for development of such wearable devices to accurately represent health conditions.
causes of death in the United States. Blood pressure (BP) is a key factor for monitoring cardiac health. Development of new optical wearable devices with capabilities to accurately measure blood pressure is becoming more prevalent and desirable. We have developed a model to generate synthetic photoplethysmographic waveforms captured by a commercial device for the radial artery at the volar surface of the wrist. This model incorporates the combination of Finite Element Model used to represent geometric changes to the radial artery and Monte Carlo light transport model to assess the interaction of optical signal with the physiological media. We highlight the effects of increasing BMI and changes attributed to obesity and epidermal melanin content on the collected PPG signal. Changes associated with obesity include trans-epidermal water loss, dermal blood content, dermal thickening, and arterial depth. We analyze the separation of PPG signal from different layers of vasculature to differentiation the signal components from the dermal plexus and main artery pulsation under 130 to 150mmHg blood pressure. Recognition of factors which effects the PPG signal is critical in accurate representation of cardiac health. Understanding changes to the PPG waveform morphology allows for development of such wearable devices to accurately represent health conditions.
Student Name: Kacie Kaile
Title: Near Infrared Imaging of Diabetic Foot Ulcers Using a Smart Phone Oxygenation Tool
co-authors: Christian Fernandez, Kevin Leiva, Wensong Wu, Maximillian Weigelt, Aliette Espinosa, Robert Kirsner, Anuradha Godavarty
Advisor: Dr. Godavarty
Lab Link: https://oil.fiu.edu
Abstract: One of the major challenges in the treatment of diabetic foot ulcers (DFUs) is patient compliance and their regular clinical visits to allow accelerated healing. Clinicians must adopt a paradigm shift from hospital and clinic care to community-based care. Virtual Medicine (VM) can greatly impact DFU wound care management with tools for remote patient monitoring (RPM). RPM shows great promise in identifying areas of impending injury/tissue loss for those who have not yet ulcerated, and the presence or absence of infection. Unfortunately, there is no low-cost mobile RPM or VM technology that can provide comprehensive (visual and physiological) clinical assessments towards diabetic wound care management and/or prevention. Herein, a novel low-cost smartphone-based imaging device has been developed to provide physiological (in terms of tissue oxygenation) and visual measurements of DFUs. The device was developed as a Smart Phone Oxygenation Tool (SPOT) containing an add-on optical module and a custom app to automate data acquisition from a multi-wavelength LED light source (650-900 nm). An IRB approved pilot study between Florida International University (FIU) and the University of Miami (UM) research hospital is currently being conducted (paused due to pandemic) using the SPOT device to map for visual and tissue oxygenation changes in DFUs and the surrounding tissues. Reduction of noise is carried out via appropriate choice of components in the hardware and via computational approaches. There is a need to implement spatial noise removal techniques for enhancing the signal collected from the smartphone detector. Herein, several noise removal techniques are explored including Single Value Decomposition (SVD), Principal Component Analysis (PCA), and Non-negative matrix factorization (NNMF). On a long-term, SPOT has potential to offer a low-cost alternative for VM and RPM in DFU wound care management.
Student Name: Steafan Khan
Title: Co-registration of Human Hand and Non-Human Primate Hand Kinematics for Invasive Brain Computer Interface Applications
co-authors: Dr. Zachary Danziger
Advisor: Dr. Zachary Danziger
Lab Link: https://anil.fiu.edu
Abstract: Invasive brain computer interfaces (iBCIs) are devices which record the spiking of cortical neurons and translate this electrical activity into commands for an assistive device such as a computer cursor or robotic arm. Our lab, in collaboration with Northwestern University, is creating a model of an iBCI to surmount the difficulty of obtaining human participants for iBCI studies. Using our model, human interaction with iBCI systems can be studied non-invasively using healthy human participants. The model will map a set of human hand kinematics to a corresponding set of non-human primate neural activity. In order to create the model, we markerlessly record non-human primate hand kinematics using the DeepLabCut software as they perform grasping tasks. We also, simultaneously record the non-human primate neural activity using intra-cortical electrode arrays. Once the non-human primate kinematics are obtained they must be translated into a qualitatively equivalent set of human hand kinematics so that the human participant’s hand gestures can be mapped to their corresponding set of primate neural activity. A Multi-Layered Perceptron (MLP) was created to perform the kinematics co-registration. The MLP took as an input non-human primate kinematics and output a corresponding set of human hand kinematics. The training dataset for the MLP was generated by isolating a set of non-human primate kinematics and by then having a human participant mimic the actions of the non-human primate hand while wearing a sensor glove. The non-human primate kinematics data was preprocessed to remove translation of the wrist and augmented via homogenous transforms to expand the set of training hand postures. Once the training data was assembled a set of candidate MLP topologies was created and a k fold cross validation was performed to identify the network topology with the best performance.
Student Name: Kevin Leiva
Title: Spatio-temporal mapping of oxygenation changes in foot ulcers
co-authors: Kacie Kaile, Valentina Roldan, Maximillian Weigelt, Aliette Espinosa, Robert Kirsner, Wensong Wu, Anuradha Godavarty
Advisor: Anuradha Godavarty
Lab Link: https://oil.fiu.edu
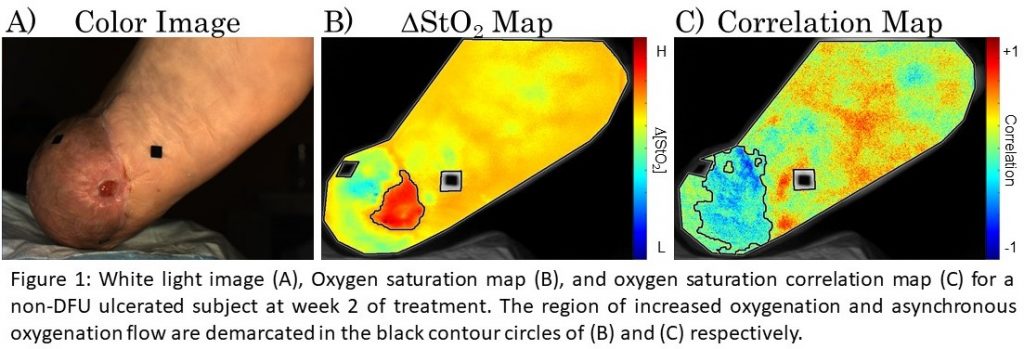
Abstract: In prior studies, it has been shown that longitudinal tissue oxygenation can be correlated to the healing status of a wound, however, potential exists in dynamic monitoring of hemoglobin-based parameters to provide additional, sub-clinical assessment of the wound healing potential. In this study, ulcerated foot subjects were monitored across 4 weeks of treatment. The objective was to monitor oxygenation changes across weeks of treatment and dynamically each session utilizing a breath-hold paradigm. Each subject was imaged via an in-house built Near InfraRed Optical Scanner (NIROS), at 690nm and 830nm, to develop spatial maps of effective hemoglobin-based parameters. Average trendlines of the effective parameters (via oxy- (∆HbO), deoxy- (∆HbR), total hemoglobin (∆HbT), and oxygen saturation (∆StO2)) were extracted. In addition, each subject was imaged with a breath-hold paradigm to calculate spatial-temporal oxygenation maps, and a Pearson’s correlation-based approach was applied to obtain correlation maps in terms of the oxygenated flow. Provided in figure 1 is the color image, oxygen saturation map, and correlation map from one subject at week 2 of treatment. Spatial oxygenation maps indicated an area of increased ∆StO2 that extended past the visible boundary of the wound. In addition, the correlation map indicated that a region of asynchronous oxygenated flow extended even further beyond the region of increased oxygenation. A Near Infrared Optical Scanner was used to conduct non-contact wound imaging of ulcerated foot subjects. NIROS has potential to assess the wound healing status and complement clinical assessments with sub-clinical physiological measurements.
Student Name: Manuel Perez
Title: Stem Cell Exosomes for Restoration of Heart Function
co-authors: Brittany Gonzalez, Yih-Mei Lin, Asad Mirza
Advisor: Dr. Sharan Ramaswamy
Lab Link: https://cvpeutics.fiu.edu
Abstract: Stem cell-injection therapy to the heart has been investigated as a viable option for the treatment of life-threatening ischemic cardiomyopathies. A major technical barrier in this field has been in prolonging stem cell survival in heart tissue, which has resulted in only marginal improvement of heart function and inconsistent outcomes between patients. However, a therapeutic agent containing the secretory constituents of stem cells (exosomes), may be used for the regenerative repair of diseased heart tissue. Our pulsatile bioreactor will be used to mechanically stimulate stem cells to produce and subsequently release exosomes in relatively high concentrations into the culture media. Demonstration of these outcomes will thereby serve as the foundation for restoration of cardiac function in vivo after treatment with our stem cell exosome therapeutic.
Objective 1 – Our current bioreactor will be used to mechanically condition stem cells seeded and exposed to physiologically-relevant oscillatory flow conditions. To evaluate the efficacy of secreted exosomes, three treatments will be investigated – (i) secretome derived from statically cultured stem cells (ii) secretome from pulsatile flow-conditioned BMSCs and (iii) the secretome derived from statically cultured cardiac stem cells which will serve as a positive control.
Objective 2 – To evaluate the benefits of our exosome treatment, a co-culture of human cardiomyocytes and fibroblasts seeded in hydrogels will be injected with our exosome product. Subsequent histological and gene expression analysis will reveal the cellular responses of cells exposed to exosomes in comparison to control samples.
Student Name: Edwin A. Robledo
Title: The impact of photon and proton therapy on tissue oxygenation in breast cancer patients
co-authors: Kevin Leiva, Corina Beiner, Juan Murillo, Maria Amelia Rodrigues, Marcio Fagundes, Joseph Panoff, Michael Chuong, Wensong Wu, Anuradha Godavarty
Advisor: Anuradha Godavarty
Lab Link: https://oil.fiu.edu
Abstract: Breast cancer has become one of the most predominant diseases among women in the U.S. and one of women’s 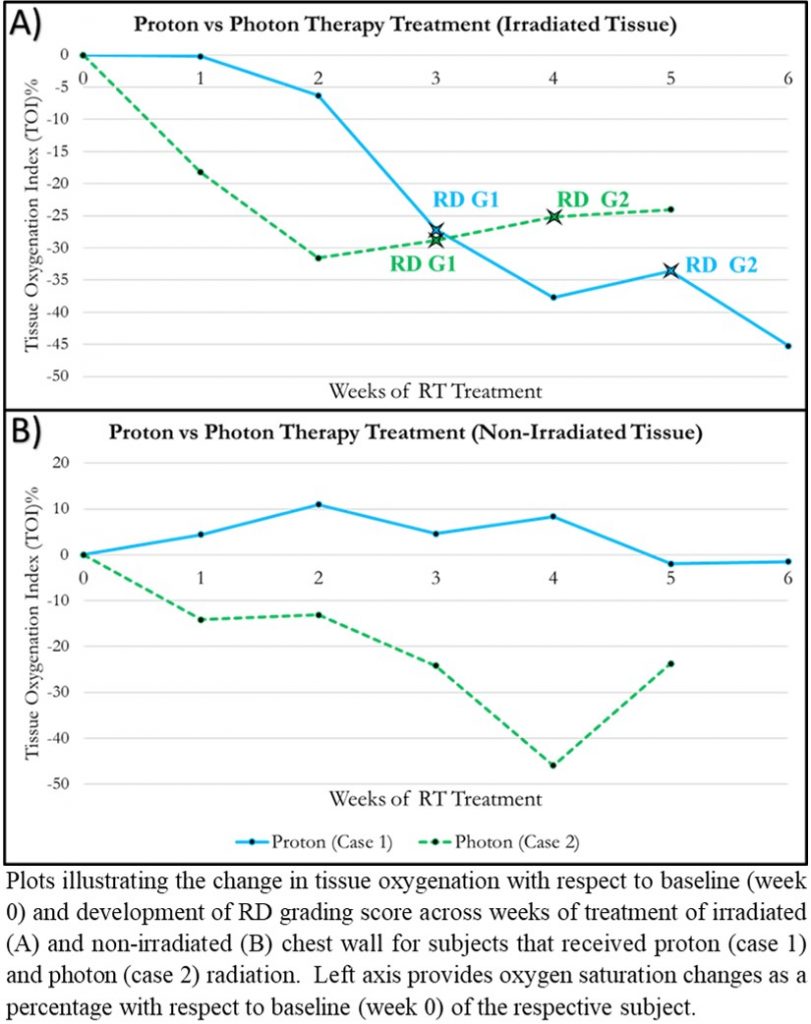 leading causes of death. The American Cancer Society has estimated that a total of 1.9 million new cancer cases will arise in 2021, 15% percent of which are breast cancer. Radiation therapy (RT) is widely used post mastectomy or lumpectomy as a method of avoiding recurrence of the disease. The main forms of RT for breast cancer patients are photon and proton therapy. The effectiveness of photon vs proton therapy has been studied by observing differences in subjective clinical grading of radiation dermatitis (RD), a common side effect of RT. This limits the studies by only observing the tissue at the surface and introduces bias from clinicians. The Optical Imaging Laboratory (OIL) at FIU is working toward an objective physiological imaging approach using near-infrared optical techniques to quantitatively differentiate the effectiveness of proton vs photon therapy by mapping the spatial and temporal changes in tissue oxygenation in breast cancer subjects undergoing RT. Past research groups have demonstrated that oxygen saturation (StO2) could serve as an early detection biomarker of skin toxicity, including characterizing and predicting onset of RD. A 6-8 week longitudinal pilot study (WIRB approved) was carried out on 10 breast cancer subjects undergoing RT at Miami Cancer Institute (MCI). From preliminary analysis, it was observed that there were distinct differences in StO2 in the irradiated regions when compared to their contralateral nonirradiated tissue. Changes in tissue oxygenation and RD were more localized and less severe in subjects receiving proton therapy compared to photon therapy. Understanding the effects of RT treatments on tissue health using a functional biomarker such as tissue oxygenation can lead to objective diagnosis and prevention of RD and other adverse events.
leading causes of death. The American Cancer Society has estimated that a total of 1.9 million new cancer cases will arise in 2021, 15% percent of which are breast cancer. Radiation therapy (RT) is widely used post mastectomy or lumpectomy as a method of avoiding recurrence of the disease. The main forms of RT for breast cancer patients are photon and proton therapy. The effectiveness of photon vs proton therapy has been studied by observing differences in subjective clinical grading of radiation dermatitis (RD), a common side effect of RT. This limits the studies by only observing the tissue at the surface and introduces bias from clinicians. The Optical Imaging Laboratory (OIL) at FIU is working toward an objective physiological imaging approach using near-infrared optical techniques to quantitatively differentiate the effectiveness of proton vs photon therapy by mapping the spatial and temporal changes in tissue oxygenation in breast cancer subjects undergoing RT. Past research groups have demonstrated that oxygen saturation (StO2) could serve as an early detection biomarker of skin toxicity, including characterizing and predicting onset of RD. A 6-8 week longitudinal pilot study (WIRB approved) was carried out on 10 breast cancer subjects undergoing RT at Miami Cancer Institute (MCI). From preliminary analysis, it was observed that there were distinct differences in StO2 in the irradiated regions when compared to their contralateral nonirradiated tissue. Changes in tissue oxygenation and RD were more localized and less severe in subjects receiving proton therapy compared to photon therapy. Understanding the effects of RT treatments on tissue health using a functional biomarker such as tissue oxygenation can lead to objective diagnosis and prevention of RD and other adverse events.
Student Name: Lin Tong
Title: Magnetic immuno-fluidic platform to identify stages of coronary artery disease and heart failure
co-authors:
Advisor: Joshua Hutcheson
Lab Link: https://hutchesonlab.fiu.edu
Abstract: Cardiovascular Disease (CVD) is the leading cause of death, especially for underserved populations due to high costs associated with treatment and lack of access to health care. Our goal is to develop a low-cost, accurate, point-of-care immuno-fluidic platform for detection of multiple biomarkers that can stratify cardiovascular risk.
Based on stages coronary artery remodeling, we have selected our current biomarker panel. Coronary artery disease begins with elevate inflammation, which can be detected by elevated circulating levels of C-Reactive Protein (CRP). Late stage coronary artery disease is characterized by the deposition of mineral in the artery wall, which is the leading predictor of cardiovascular morbidity. We have previously shown that serum levels of the protein Sortilin correlates with arterial mineral and predicts cardiovascular events over the course of 8 years. Finally, cardiac Troponin I (cTnI) is elevated in serum during heart failure. The low circulating concentration of cTnI (10 to 30 pg/mL) makes it particularly difficult to measure in serum. We have demonstrated the capability to reach a cTnI detection limit of 4.3 pg/mL and detection range of 4.3~125 pg/mL using a colorimetric method. We have also developed an advanced data analysis technique to improve the system robustness, detection range, and assay time. A soft lithography based fluidic platform is currently under development for point-of-care use. Overall, we seek to develop a low cost, accurate, and easy to use device for cardiovascular biomarker detection.
- 10 Ariadna Herrera
- 11 Beatriz Herrera
- 12 Xiang Kong
- 13 Yih-Mei Lin
- 14 Asad Mirza
- 15 Carolina Moncion
- 16 Andres J. Rodriguez
- 17 Alejandro Suarez
- 18 Sk Yeahia Been Sayeed
Student Name: Ariadna Herrera
Title: Comparison of Engineered Cardiac Tissue and Native Myocardium Mechanical Properties
co-authors: Yih-Mei Lin, Lihua Lou, Arvind Agarwal, Sharan Ramaswamy
Advisor: Sharan Ramaswamy
Lab Link: https://cvpeutics.fiu.edu
Abstract: Cardiac disease is a leading cause of death worldwide. For example, in the United States, it is the number one cause of death, with myocardial infarction being a predominant condition. Heart tissue does not have the ability to regenerate itself, and current medical treatments are unable to promote do novo cardiac tissue formation. Thus, engineered cardiac tissue may be a treatment strategy for myocardial infarction. To engineer cardiac tissue, iPSC-Cardiomyocytes are first seeded on 3D scaffolds. The constructs need to then be appropriately conditioned under conducive biochemical, biomechanical and/or bioelectrical conditions. To have optimal, functional engineered cardiac patches for repair, the scaffold material should have mechanical properties similar to that of native heart tissue. In order to select the scaffold that will best fit these requirements, we will conduct mechanical testing on two different scaffolds, RTV Silicone with a double-strand design and a bioscaffold known as Porcine Small Intestinal Submucosa (PSIS), to compare their mechanical properties to our positive control (fresh porcine left ventricular myocardium). Uniaxial tensile tests will be performed to obtain the constitutive properties and the Young’s Modulus of each material. It is expected that the results of this study will allow us to choose the more ideal scaffold for seeding iPSC-Cardiomyocytes and continue developing engineered cardiac tissue that can potentially be used to treat damaged heart tissue due to cardiac diseases.
Student Name: Beatriz Herrera
Title: Assessing the validity of the EEG dipolar approximation in rats, monkeys, and humans: a computational modeling study.
co-authors: Jeffrey D. Schall, Jorge J. Riera
Advisor: Jorge J. Riera
Lab Link: https://nmd.fiu.edu
Abstract: Electroencephalography (EEG) is among the most widely used brain imaging techniques for non-invasively studying 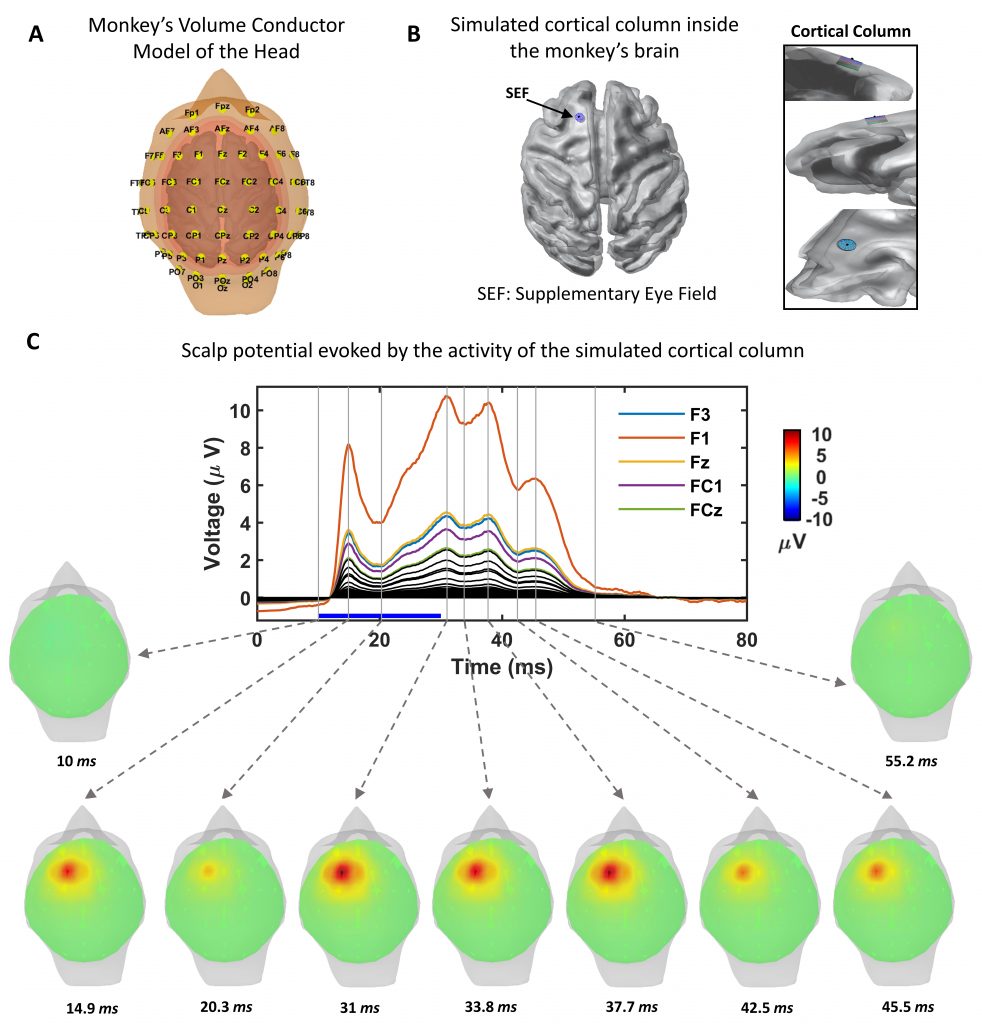 brain cognition and pathologies. These signals are known to originate from cortical neuronal activity and, for decades, have been described in terms of current dipoles. The dipolar approach has been the basis of the EEG inverse methods to estimate the brain sources giving rise to the scalp potentials in both research and clinical applications. The basic assumption of the EEG dipolar approach is that the recording location is far enough from the neuronal sources originating the measured signals. Given the differences in head’s shape and dimension across species, a quantitative assessment of the validity of the dipolar model is necessary. In the present work, we use detailed biophysical modeling to quantify the error made by assuming the dipolar model in rats, monkeys, and humans. We simulated the activity of a cortical column consisting of 1,000 layer 5 pyramidal cells under suprathreshold stimulation located in cognitive control areas in the rat, monkey, and human brain. The EEG resulting from their activation was estimated, using the detailed head model for each species, considering the contribution of each neuron, and using the dipolar approximation. We found that the error made using the dipolar model is greater in rats than in monkeys and humans, having the latest the smallest error. Additionally, the error for columns located in more superficial areas was bigger than those in deeper areas, farther from the electrodes.
brain cognition and pathologies. These signals are known to originate from cortical neuronal activity and, for decades, have been described in terms of current dipoles. The dipolar approach has been the basis of the EEG inverse methods to estimate the brain sources giving rise to the scalp potentials in both research and clinical applications. The basic assumption of the EEG dipolar approach is that the recording location is far enough from the neuronal sources originating the measured signals. Given the differences in head’s shape and dimension across species, a quantitative assessment of the validity of the dipolar model is necessary. In the present work, we use detailed biophysical modeling to quantify the error made by assuming the dipolar model in rats, monkeys, and humans. We simulated the activity of a cortical column consisting of 1,000 layer 5 pyramidal cells under suprathreshold stimulation located in cognitive control areas in the rat, monkey, and human brain. The EEG resulting from their activation was estimated, using the detailed head model for each species, considering the contribution of each neuron, and using the dipolar approximation. We found that the error made using the dipolar model is greater in rats than in monkeys and humans, having the latest the smallest error. Additionally, the error for columns located in more superficial areas was bigger than those in deeper areas, farther from the electrodes.
Student Name: Xiang Kong
Title: Artificial Intelligence-Assisted Monte Carlo based voxel dose calculation for thyroid remnant ablation
co-authors: Seza Gulec, Xiaodong Wu, Mike Georgiou, Malek Adjouadi, Wei-Chiang Lin
Advisor: Anthony McGoron
Lab Link:
Abstract: Thyroid nodules are a common clinical problem. According to American Thyroid Association guideline, a total thyroidectomy is the first line option for thyroid nodules management. Detectable functioning thyroid tissue (remnants) are often left behind due to surgical and practical considerations. Radioactive iodine (RAI) therapy utilizes radioactive iodine (I-131) to ablate the thyroid remnant.
Historically the treatment strategy for RAI remnant ablation is based on a fixed activity regiment. Typically, a diagnostic imaging study (SPECT with I-131 or PET with I-124) with small amount of I-131/I-124 is performed prior to determine the patient’s eligibility and dose selection (50mCi for low-risk patients post operation and 100mCi for high-risk patients). With the evolution of precision medicine, personalize dosimetry planning and evaluation should be adapted. There is a large body of clinical evidence indicating the potential value of dosimetric evaluation in patients undergoing RAI therapy. The scientific and intellectual basis of RAI dosimetry has been established but not clinically utilized yet. In this project, we are trying to improve the current RAI dosimetry by using modern AI method. More accurate dosimetry prediction can be expected and this could change the RAI practice for the future.
Clinical SPECT or PET images suffer from the low spatial resolution (5mm pixel spacing). This puts limits on both diagnostic and dosimetry value of the RAI imaging studies. With the development of modern artificial intelligence, we are proposing to reconstruct the images and upscale the resolution to 1mm using machine learning method (Neural Network).
Phantom SPECT/CT imaging studies with I-131 will be performed starting with clinical concentration (50μCi/cc) and different target volumes (1cc to 0.1cc). This could provide us the remnant detectability information from both activity concertation level and size aspect. A series of SPECT and CT scans will be acquired, which can also be used as training data set for model generation.
The volumetric dose calculation is done using 3D convolution with dose deposition kernel. The current published 5mm dose kernel can no longer provide precision dosimetry information for target dose coverage and evaluation. High resolution dose kernel (1mm) can be calculated from Monte Carlo simulation (MCNP, FLUKA or Geant4). More meaningful dosimetry information can be achieved.
Several previously treated thyroid remnant patients’ images are available for analysis. Retrospective study results will provide useful information for future guidance.
Student Name: Yih-Mei Lin
Title: Improvement of Human iPSC-Cardiomyocytes-Derived Tissue Maturation Under Flex-Stretch Environments
co-authors: Yih-Mei Lin, Ariadna Herrera, Lihua Lou, Arvind Agarwal, Sharan Ramaswamy
Advisor: Dr. Sharan Ramaswmay
Lab Link: https://cvpeutics.fiu.edu
Abstract: Human induced pluripotent stem cells (hiPSC) and the generation of human induced pluripotent stem cells-derived cardiomyocytes (hiPSC-CM) have been considered as a promising aspect to regenerating cardiac tissues. The hiPSC-CM technology can be developed and utilized to repair or replace damaged cardiac tissue due to heart disease. However, current techniques showed limited results to efficiently generate mature cardiomyocytes. The functional maturation of cardiomyocytes is a desired phenotype but this is challenging to achieve and sustain in an in vitro environment. The incorporation of a scaffold in 3D culture can facilitate a more similar in vivo extracellular microenvironment that supports better cellular interactions. As such, we propose a robust 3D culture system with the application of mechanical cues including flex and stretch in a bioreactor. In addition to the mechanical stimulation, the use and selection of tissue scaffolds will also be taken into consideration. Two of the engineered tissue scaffolds will be tested with and without cells: RVT silicone and porcine small intestine submucosa (PSIS). In order to mimic physiologically-relevant artery system, a 1/3 of flexing/cycle and 2/3 of stretching/cycle protocol with 1.2Hz frequency (72 beats/minute) will be applied to the scaffolds as a starting point. The engineered scaffolds will be histologically compared with the native porcine myocardium that serves as a positive control in the study. These findings should provide a comprehensive understanding on how flex-stretch mechanical stimulation can promote the maturation of hiPSC-CM-derived engineered cardiac tissue formation.

Student Name: Asad Mirza
Title: Enhanced Hemodynamics Predictions in a Calcified Aortic Valve Geometry Using the Quemada Model
co-authors: Amanda Barreto, Tisha Boodooram, Sharan Ramaswamy
Advisor: Dr. Sharan Ramaswamy
Lab Link: https://cvpeutics.fiu.edu
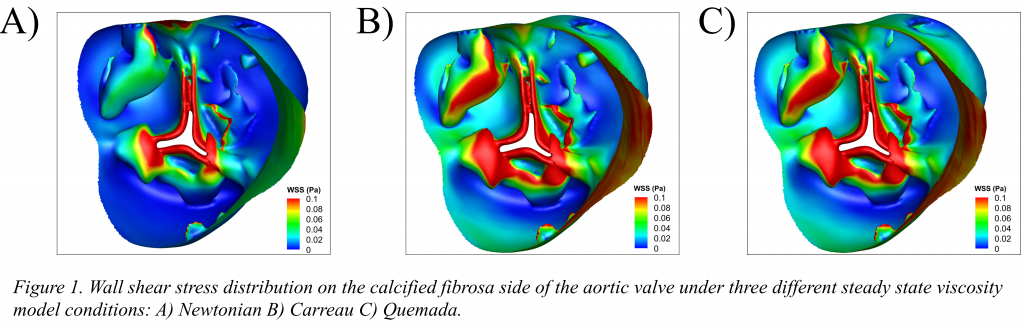
Abstract: When conducting computational fluid dynamics (CFD) simulations around the aortic valve, blood is often assumed to be a Newtonian fluid. This assumption is supported through previous studies of similar large vessels in arteries where shear rates are relatively large, (>1000 s-1). However, for the aortic valve, leaflet flexure and calcifications can induce regions of very low shear rates on the fibrosa side which could be prone to thrombogenesis. The following study investigated and compared three viscosity models: Newtonian, Carreau, Quemada on a calcified aortic valve geometry to determine which model would facilitate maximum accuracy in hemodynamic predictions. Geometry of a human aortic heart valve at the early diastolic phase was processed for mesh repair, smoothing, shrink-wrap, labelling, and fluid volume extraction using ANSYS software. Inlet velocity of 1 cm/s along with an 80-mmHg pressure condition on the outlet were prescribed. Blood flow was assumed to be incompressible and modeled using the Navier-Stokes equations. Blood viscosity, µ, was defined using three different models: Newtonian, Carreau, and Quemada with parameter values being found by curve fitting experimental blood data using a non-linear least squares algorithm in MATLAB. Within the cusp regions WSS was often greater under both non-Newtonian models (> 0.4 dynes/cm2), than in the same area under the Newtonian model. The Quemada and Carreau models exhibited an excellent response across a wide range of shear rates. However, the Quemada model has the added benefit of accounting for changes in blood hematocrit, a parameter that has been noted to vary with both age and gender of patients. We conclude that that the Quemada model is most applicable for calcified aortic valve geometries so as to capture leaflet WSS more accurately and to identify regions of flow stagnation, which are vulnerable to thrombosis.
Student Name: Carolina Moncion
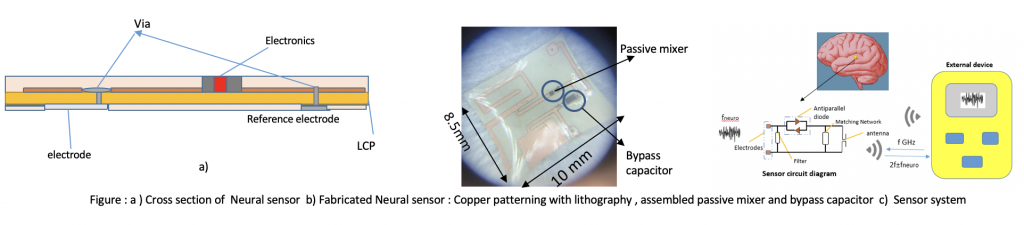
Title: Wireless Neurosensing System for Recording Multi-unit Neuronal Activity
co-authors: Lakshmini Balachandar, Satheesh Bojja-Venkatakrishnan, Asimina Kiourti, John L. Volakis, and Jorge Riera Diaz
Advisor: Jorge Riera
Lab Link: https://nmd.fiu.edu
Abstract: Intracranial techniques for electrophysiological recording are crucial in assessing normal and pathological neuronal activity. Signal activity can be collected to represent groups of neurons (electrocorticography, ECoG, and local field potentials, LFP) or individual (neuronal spiking) levels, each offering invaluable information about the brain’s functioning. To better assess the normal operation of the brain’s functioning wireless recorders have been proposed. However, current wireless systems are generally bulky as they require the use of integrated power sources that may lead to heat-generating tissue-damage.
To remove the need for batteries in existing neural sensors, we proposed a passive, wireless, and fully implantable next-generation wireless neurosensing system (WiNS). We have already performed initial in vivo validation for recording evoked activation, equivalent of ECoG. This device consists of an implant and interrogator antenna, and electrodes integrated with a demodulation circuit. Recently, WiNS has been incorporated with an impedance matching network to address mismatches between the neural probes and recording circuits. The impedance matching network includes a Schottky diode connected to the electrodes through a bipolar junction transistor to function as an impedance buffer. This adaptation along with a novel impedance reducing electrochemical coating on the microelectrodes allowing for strong recording of individual neuronal spikes, complementing our earlier population recordings.
Building on our WiNS system validation to date, we set out to conduct a study to record the individual activity of multiple individual neurons. Achieving this goal, combined with our previous accomplishments demonstrates that WiNS can be applied for multiscale neuronal activity monitoring. We will present recordings of spontaneous neuron activity from the somatosensory cortex and hippocampus. Our recordings have also sensed evoked activity in these regions following electrical hind limb stimulation in rats. All in all, a preliminary analysis indicates that WiNS will greatly impact future neuroscience research and lead to practical clinical options.
Student Name: Andres J. Rodriguez
Title: optical properties in the obese skin: the application of spatial frequency domain spectroscopy (SFDS)
co-authors: Ajmal, Mariacarla Gonzalez, Tananant “Mel” Boonya-ananta, Vinh Nguyen Du Le, Dr. Jessica C. Ramella-Roman
Advisor: Dr. Jessica C. Ramella-Roman
Lab Link: https://web.eng.fiu.edu/jramella/
Abstract: Obesity is an epidemic that increases risk of cardiovascular diseases. Wearable devices can measure cardiac 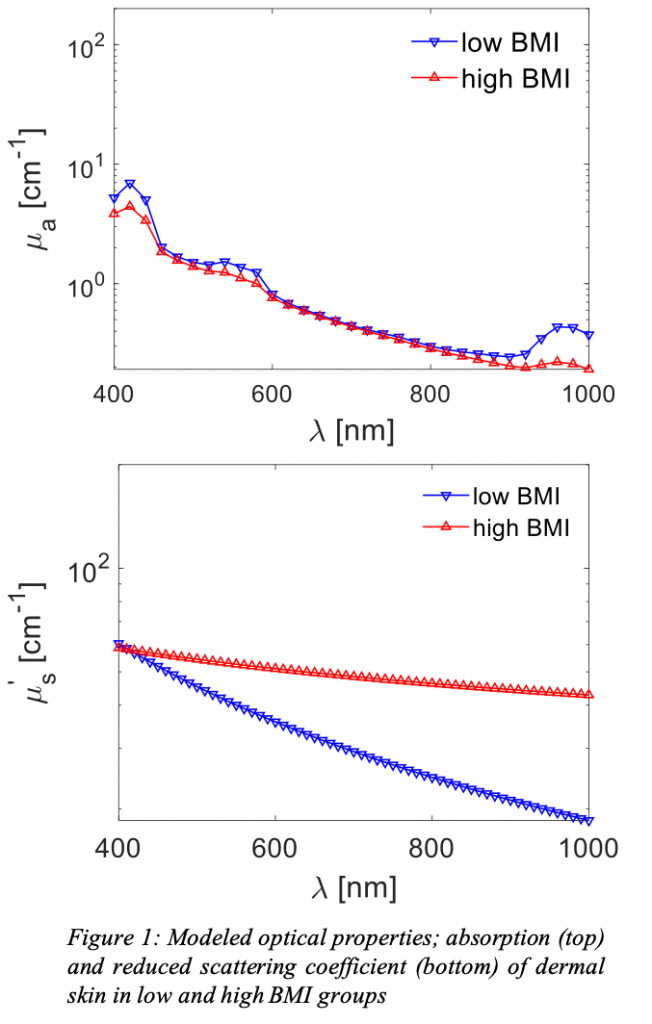 biomarkers; including heart rate, heart rate variability, perfusion, pressure pulse-wave velocities, among others. Most wearables using optical sensors rely on fluctuations due to spatio-temporal variations in tissue absorption. Individuals with high Body Mass Index (BMI) have thicker adipose tissues that scatter the signal, yielding poorer optical contrast and SNR. Moreover, higher BMI alters chemical concentrations— like water, oxygenation, and blood volume in the dermal layer— and thus the optical properties (OPs). Although OPs of the skin exist in literature, no study has recorded the effect and magnitude of higher BMI. We aim at constructing an imaging device to quantify skin OPs. We hypothesize individuals with higher BMI will show significant changes in OPs. Our data acquisition will rely on Spatial Frequency Domain Spectroscopy (SFDS). SFDS separates and quantifies the absorption coefficient and reduced scattering coefficient as a function of wavelength by acquiring phase-shifted sinusoidal projections, signal demodulation, model calibration, and finally solving an inverse problem. We will assemble, calibrate, and verify the workings of an SFDS system on phantoms. The OPs calculated should have insignificant error differences from phantoms’ known properties. The system will be made ready for in-clinic use to acquire the OP of larger cohorts. A larger study will aid in understanding how the anatomical and physiological changes in the obese skin affects the use optical sensors that record physiological biomarkers. Future works will minimize system and enhance portability for cross-institutional deployment.
biomarkers; including heart rate, heart rate variability, perfusion, pressure pulse-wave velocities, among others. Most wearables using optical sensors rely on fluctuations due to spatio-temporal variations in tissue absorption. Individuals with high Body Mass Index (BMI) have thicker adipose tissues that scatter the signal, yielding poorer optical contrast and SNR. Moreover, higher BMI alters chemical concentrations— like water, oxygenation, and blood volume in the dermal layer— and thus the optical properties (OPs). Although OPs of the skin exist in literature, no study has recorded the effect and magnitude of higher BMI. We aim at constructing an imaging device to quantify skin OPs. We hypothesize individuals with higher BMI will show significant changes in OPs. Our data acquisition will rely on Spatial Frequency Domain Spectroscopy (SFDS). SFDS separates and quantifies the absorption coefficient and reduced scattering coefficient as a function of wavelength by acquiring phase-shifted sinusoidal projections, signal demodulation, model calibration, and finally solving an inverse problem. We will assemble, calibrate, and verify the workings of an SFDS system on phantoms. The OPs calculated should have insignificant error differences from phantoms’ known properties. The system will be made ready for in-clinic use to acquire the OP of larger cohorts. A larger study will aid in understanding how the anatomical and physiological changes in the obese skin affects the use optical sensors that record physiological biomarkers. Future works will minimize system and enhance portability for cross-institutional deployment.
Student Name: Alejandro Suarez
Title: Identification of Negative BOLD Responses in Epilepsy using Windkessel Models
co-authors: Pedro A. Valdés-Hernández, PhD; Byron Bernal, MD; Catalina Dunoyer, MD; Hui Ming Khoo, MD, PhD, Jorge Bosch-Bayard, PhD; MD, PhD; Nicolas von-Ellenrieder, PhD; Jean Gotman, PhD; Jorge J. Riera, PhD
Advisor: Dr. Jorge Riera
Lab Link: Neuronal Mass Dynamics Laboratory (https://nmd.fiu.edu)
Abstract: Alongside positive BOLD responses (PBR) associated with interictal epileptic discharges, a variety of negative BOLD responses (NBR) with distinct underlying mechanisms are typically found in epileptic patients. Previous studies suggest that, in general, up to four mechanisms might underlie the genesis of NBRs in the brain: i) neuronal disruption of network activity (NDA), ii) altered balance of neuro-metabolic/vascular couplings (ANC), iii) arterial blood stealing (ABS), and iv) enhanced cortical inhibition (ECI). Detecting and classifying these mechanisms from BOLD signals is pivotal for the improvement of the specificity of the EEG-fMRI image modality to identify the seizure onset zones in refractory local epilepsy. This requires models with physiological interpretation that furnish the understanding of how these mechanisms are fingerprinted by their BOLD responses. Here, we used a windkessel model with viscoelastic compliance/inductance in combination with dynamic models of both neuronal population activity and tissue/blood O2 to classify the hemodynamic response functions (HRF) linked to the above mechanisms in the irritative zones of epileptic patients. Firstly, we evaluated the most relevant imprints on the BOLD response caused by variations of key model parameters. Secondly, we demonstrated that a general linear model is enough to accurately represent the four different types of NBRs. Thirdly, we tested the ability of a machine learning classifier, built from a simulated ensemble of HRFs, to predict the mechanism underlying the BOLD signal from irritative zones. Crossvalidation indicates that these four mechanisms can be classified from realistic fMRI BOLD signals. Finally, we successfully applied our methodology to EEG-fMRI data from five epileptic patients undergoing neurosurgery where some of these mechanisms coexisted. We concluded that a proper identification and interpretation of NBR mechanisms can be performed by combining general linear models and biophysically-inspired models.
Student Name: Sk Yeahia Been Sayeed
Title: Miniaturized Health Monitoring Device with Low cost, Light weight Advanced 3D High Density Heterogenous Flexible Packaging
Advisor: John Volakis, PM Raj
Lab Link:
Abstract: Recent development in sensing electrode interfaces, single-chip signal processing and modulation, wireless power and communication, and fabrication technologies has enabled low power devices and miniaturization with unparalleled interfacing and functionalities to biological tissue in complex physiological environment. More importantly, understanding vital human organs’ complex functionalities has improved considerably, leading to major advances in neuroscience and cardiovascular therapies. Development of wireless neural recording devices or engineer efficient devices (less than 10 mm) that function satisfactorily under scalp, skull, and tissue while animals involve in regular activities has been a perennial challenge in translating neurotechnologies to clinical practice. Tissue is soft and curved, so the whole device packaging should be flexible and biocompatible. As devices get miniaturized, particularly below 10 mm, providing adequate power becomes a hurdle. Since IC’s(Integrated circuit), power telemetry devices, passive components must be assembled properly in a system-level approach. Thus, Heterogenous integration in flexible package is vital. To address these challenges , we introduce -1) miniaturized planar antenna 2) Co-packaging planar antenna with MMIC and passive component with fully additive manufacturing technique 3) High impedance Circuit architecture to detect high resolution neuropotential 4) Zero/ low power miniaturized , weight less than a single grain of rice, flex neural sensor 5) Multimodal power and data telemetry with a combination Magnetoelectric composite.
- 19 Ajmal Ajmal
- 20 Amirala Bakhshiannik
- 21 Lakshmini Balachandar
- 22 Arezoo Geramipour
- 23 Mariacarla Gonzalez
- 24 Denise Hsu
- 25 Mohammad Shaver
- 26 Anil Thota
Student Name: Ajmal
Title: Investigation of optical heart rate sensors in wearables and the influence of skin tone and obesity on Photoplethysmography (PPG) signal
co-authors: Mel Tananant Boonya-ananta, Andres J. Rodriguez, Vinh Nguyen Du Le, Jessica C. Ramella-Roman
Advisor: Jessica C Ramella-Roman
Lab Link: https://web.eng.fiu.edu/jramella/
Abstract: The ability of wearables to provide continuous monitoring of heart rate makes them particularly suitable for the  management of chronic health conditions, such as diabetes and cardiovascular diseases, outside the clinic. Heart rate measurement with wearable devices is accomplished by a non-invasive photometric technique called Photoplethysmography (PPG)-based sensors, through quantifying blood volume change. The prime source of PPG signals, in case of common wearables, is the volumetric change in blood in the dermal vasculature. Here we present a novel approach of using a more realistic skin model containing a double vascular layer within the dermis to investigate the pulsatile contribution from the region. Finite Element Method (FEM) is used to design vessels, with characteristic biomechanical behavior, that represent pulse pressure from these lower and upper blood networks of dermis. The motivation of our work, through Monte Carlo modeling, is to study light transport within skin tissue and to effectively extract the PPG waveform from the wrist. In this aspect, we performed the assessment of the hardware architecture of PPG sensors in some of the commercially available optical wearable devices and their corresponding PPG waveforms are investigated. We studied the influence of skin tone and obesity on the PPG signals, where a difference in pulse signals is observed for varying Body Mass Index (BMI).
management of chronic health conditions, such as diabetes and cardiovascular diseases, outside the clinic. Heart rate measurement with wearable devices is accomplished by a non-invasive photometric technique called Photoplethysmography (PPG)-based sensors, through quantifying blood volume change. The prime source of PPG signals, in case of common wearables, is the volumetric change in blood in the dermal vasculature. Here we present a novel approach of using a more realistic skin model containing a double vascular layer within the dermis to investigate the pulsatile contribution from the region. Finite Element Method (FEM) is used to design vessels, with characteristic biomechanical behavior, that represent pulse pressure from these lower and upper blood networks of dermis. The motivation of our work, through Monte Carlo modeling, is to study light transport within skin tissue and to effectively extract the PPG waveform from the wrist. In this aspect, we performed the assessment of the hardware architecture of PPG sensors in some of the commercially available optical wearable devices and their corresponding PPG waveforms are investigated. We studied the influence of skin tone and obesity on the PPG signals, where a difference in pulse signals is observed for varying Body Mass Index (BMI).
Student Name: Amirala Bakhshiannik
Title: Targeting Epidermal Growth Factor Receptor in Vascular Calcification
co-authors: Hooi Hooi Ng, Joshua D. Hutcheson
Advisor: Dr. Joshua D. Hutcheson
Lab Link: https://hutchesonlab.fiu.edu
Abstract: Medial calcinosis manifests as the mineralization of the medial layer of vessel walls, leading to vascular stiffening,  dysfunction, and cardiac overload. Calcification of arterial media commonly occurs in patients with chronic kidney disease (CKD). CKD patients with no detectable vascular calcification have 8-year all-cause survival rates of ~90% compared to 50% survivability in age-matched patients with medial calcification. Calcifying extracellular vesicles (EVs) mediate mineral nucleation and growth by stabilizing calcium and phosphate interactions. The biogenesis of these EVs from vascular smooth muscle cells (VSMCs) requires the presence of caveolin-1 (CAV1), the major scaffolding protein within plasma membrane invaginations, known as caveolae. Epidermal growth factor receptor (EGFR) interactions with CAV1 modulates EGFR-mediated signaling pathways and caveolae trafficking. However, the role of EGFR in vascular calcification has not been addressed. EGFR inhibitors are clinically approved and used in cancer therapies; therefore, demonstrating a role for EGFR in vascular calcification could establish the first therapy for vascular calcification. Our results showed that EGFR inhibition significantly reduced vascular calcification in a CKD mouse model compared to non-treated controls (p = 0.003). The observed reduction in vascular calcification by EGFR inhibition correlated with reduced release of CAV1-positive EVs with elevated mineral-promoting factors into the vascular wall (p = 0.001, compared to non-treated controls). In vitro experiments with isolated human coronary artery VSMCs also show that EGFR inhibition prevents mineral formation in osteogenic culture conditions. EGFR inhibitor treatment in vitro prevented the release of calcifying EVs by modulating intracellular trafficking of CAV1 without affecting the phenotype of these cells. Interestingly, we also showed that EGFR inhibition does not affect the in vitro mineralization of human osteoblasts. Given the specific effect on pathological, but not physiological mineralization, these data suggest that EGFR inhibition may provide the first therapeutic option for vascular calcification.
dysfunction, and cardiac overload. Calcification of arterial media commonly occurs in patients with chronic kidney disease (CKD). CKD patients with no detectable vascular calcification have 8-year all-cause survival rates of ~90% compared to 50% survivability in age-matched patients with medial calcification. Calcifying extracellular vesicles (EVs) mediate mineral nucleation and growth by stabilizing calcium and phosphate interactions. The biogenesis of these EVs from vascular smooth muscle cells (VSMCs) requires the presence of caveolin-1 (CAV1), the major scaffolding protein within plasma membrane invaginations, known as caveolae. Epidermal growth factor receptor (EGFR) interactions with CAV1 modulates EGFR-mediated signaling pathways and caveolae trafficking. However, the role of EGFR in vascular calcification has not been addressed. EGFR inhibitors are clinically approved and used in cancer therapies; therefore, demonstrating a role for EGFR in vascular calcification could establish the first therapy for vascular calcification. Our results showed that EGFR inhibition significantly reduced vascular calcification in a CKD mouse model compared to non-treated controls (p = 0.003). The observed reduction in vascular calcification by EGFR inhibition correlated with reduced release of CAV1-positive EVs with elevated mineral-promoting factors into the vascular wall (p = 0.001, compared to non-treated controls). In vitro experiments with isolated human coronary artery VSMCs also show that EGFR inhibition prevents mineral formation in osteogenic culture conditions. EGFR inhibitor treatment in vitro prevented the release of calcifying EVs by modulating intracellular trafficking of CAV1 without affecting the phenotype of these cells. Interestingly, we also showed that EGFR inhibition does not affect the in vitro mineralization of human osteoblasts. Given the specific effect on pathological, but not physiological mineralization, these data suggest that EGFR inhibition may provide the first therapeutic option for vascular calcification.
Student Name: Lakshmini Balachandar
Title: Simultaneous Ca2+ Imaging and Optogenetic Stimulation of Cortical Astrocytes in Adult Murine Brain Slices
co-authors: Lakshmini Balachandar, Carolina Moncion, Karla A. Montejo, Eleane Castano, Melissa Perez, Jeremy W. Chambers, J. Luis Lujan, and Jorge Riera Diaz
Advisor: Dr. Jorge Riera Diaz
Lab Link: https://nmd.fiu.edu
Abstract: Astrocytes modulate neuroinflammation and reactive gliosis prevalent in several brain disorders like epilepsy, Alzheimer’s, and Parkinson’s disease. In animal models, targeted manipulation of astrocytic function via modulation of their calcium (Ca2+) oscillations by incorporating light‐sensitive cation channels like Channelrhodopsin‐2 (ChR2) offers a promising avenue in influencing the long‐term progression of these disorders. However, using adult animals for Ca2+ imaging poses major challenges, including accelerated deterioration of in situ slice health and age‐ related changes. Additionally, optogenetic preparations necessitate usage of a red‐shifted Ca2+ indicator like Rhod‐2 AM to avoid overlapping light issues between ChR2 and the Ca2+ indicator during simultaneous optogenetic stimulation and imaging. To this end, we provide an experimental setting that uses live adult murine brain slices (2‐5 months) from a knock‐in model expressing ChR2(C128S) in neocortical astrocytes, loaded with Rhod‐2 AM to elicit robust Ca2+ response to light stimulation. We have developed and standardized a protocol for brain extraction, sectioning, Rhod‐2 AM loading, maintenance of slice health, and Ca2+ imaging during light stimulation. This has been successfully applied to optogenetically control adult cortical astrocytes, exhibited synchronous patterns of Ca2+ activity upon light stimulation, drastically different from resting spontaneous activity and based on the effect of various light paradigms, we identified those conducive for robust astrocytic signaling.
Student Name: Arezoo Geramipour
Title: Loss of Urethral Sensitivity Leads to Functional Deficits in Rat Model: Implications for Age Related Underactive Bladder
co-authors: Zachary Danziger
Advisor: Zachary Danziger
Lab Link:
Abstract: Age
related underactive bladder (UAB) affects 10% of elderly people in the U.S., has high associated healthcare expenses, and leads to many lower urinary tract dysfunctions. Age related UAB remains difficult to treat because its underlying causes are still
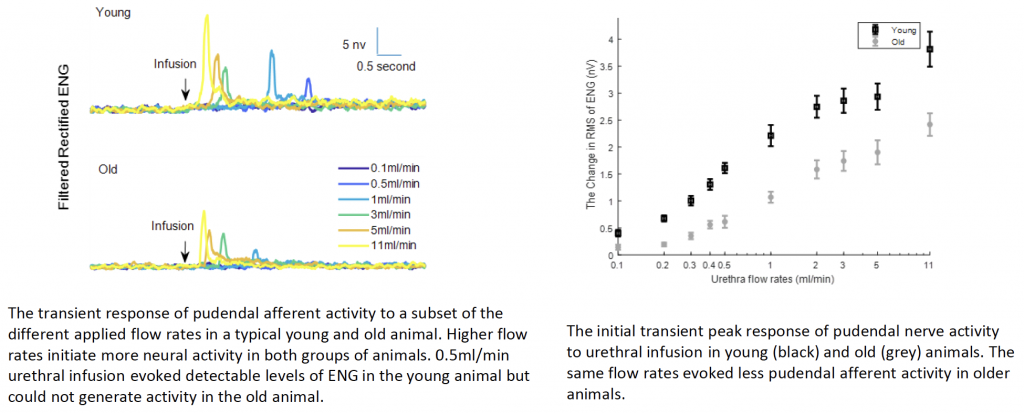
unknown. Our previous work showed that voiding reflexes evoked by urethral sensation are weaker in older animals. That motivates us to hypothesize that the sensitivity of urethral afferents decreases with age, which could lead to a disruption of the voiding reflexes required to establish efficient voiding. To test this, we measure directly the urethral afferents activity to investigate if urethral signaling weakens with age and if this loss of sensitivity drives reduced reflex function. We measure the pudendal afferent response in young (4 7 months) and old (18 24months) rats to fluid flow in the urethra across a range of flow rates. Paraffin embedding and H&E staining are used to quantify age related changes in the sensory branch of the pudendal nerve. The results show that urethra afferent signaling in response to same urethral flow rates is weaker in older animals. Therefore, the signaling reduction of urethra afferents to flow may weaken the functionality of augmenting reflex and contribute to incomplete bladder emptying in elderly population. Furthermore, the same flow rates evoked less pressure in the urethra
in old animals, indicating that the urethra muscle compliance decreases with age. Since urethral pressure is the putative driver of urethral afferents, the reduced flow evoked urethral pressure can decrease the activity of urethral afferents in response to urethral flow.
Student Name: Mariacarla Gonzalez
Title: 4×3 Mueller matrix imager for health diagnosis
co-authors:
Advisor: Jessica Ramella-Roman
Lab Link: https://web.eng.fiu.edu/jramella/
Abstract: Invasive cervical cancer is a slow progressing disease, taking more than 10 years to fully develop from infection. The anatomic accessibility and possible treatment of precancerous lesions make early screening a suitable and effective management. The standard procedure for cervical testing includes cytology (Pap test), followed by colposcopy, biopsy and histological confirmation. This procedure, however, requires a high level of quality standards such as trained personnel, medical coverage and follow up visits. A portable, low cost device that provides quantitative information on cervical health and is user-independent is needed. An imager based on Mueller matrix polarimetry consisting of a polarization state generator of four input polarization states and a polarization state analyzer on board of the detector, a polarized camera, is introduced. A 4×3 reduced form of the Mueller matrix is decomposed to extract polarimetric parameters such as the retardance, depolarization and orientation. The use of this device on several samples is tested.
Student Name: Chia-Pei Denise Hsu
Title: Tricuspid versus Mitral Performance of Cylindrical Porcine Small Intestinal Submucosa Valves
co-authors: Asad Mirza, Robert Matheny, Sharan Ramaswamy
Advisor: Sharan Ramaswamy
Lab Link: https://cvpeutics.fiu.edu
Abstract: Objective:
Treatment options are extremely limited for children and older patients who are contra-indicated for receiving mechanical or 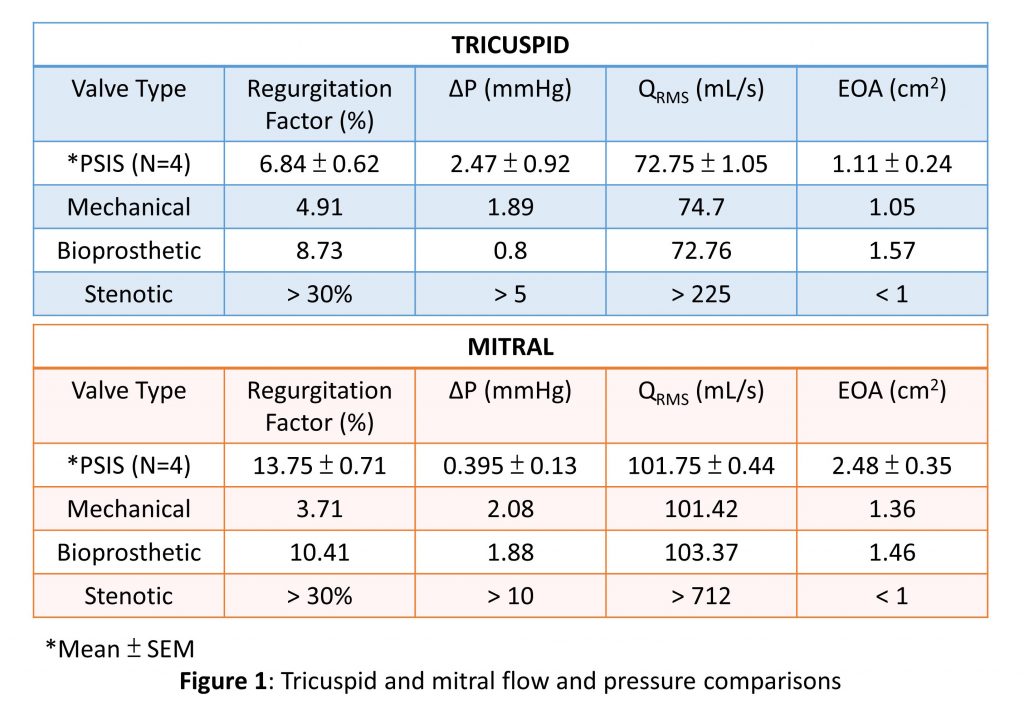 bioprosthetic valves. The purpose of this study is to determine whether cylindrical porcine small intestinal submucosa (PSIS) bio-scaffold valves can facilitate robust valvular function in the mitral after having undergone recent clinical experience in the tricuspid location.
bioprosthetic valves. The purpose of this study is to determine whether cylindrical porcine small intestinal submucosa (PSIS) bio-scaffold valves can facilitate robust valvular function in the mitral after having undergone recent clinical experience in the tricuspid location.
Methods:
A 26-mm PSIS cylindrical valve (CorMatrix) was sutured to a 3D printed valve holder along three posts at 120 degrees apart on the distal end. The annulus ring was sealed with a 3D printed cap. Hydrodynamic testing of valves mounted in mitral and tricuspid positions were performed using a pulse duplicator system (Vivitro) with 0.9% saline solution. A flow probe was affixed between atrium and ventricular chambers, and pressure transducers were inserted in the atrial, ventricular, and aortic locations. Tests utilized heart rate of 70 BPM, an input waveform comprising of a 35% systolic-65% diastolic configuration (Vivitest), and a stroke volume of 50 mL for tricuspid and 71.4 mL for mitral conditions.
Results:
Valve performance in the tricuspid position showed root mean square volumetric flow rate (QRMS) of 72.75 mL/s, transvalvular pressure gradient (ΔP) of 2.47 mmHg, effective orifice area (EOA) of 1.11 cm2, and regurgitation factor (RF) of 6.84%. In the mitral position, the results showed QRMS of 101.75 mL/s, ΔP of 0.395 mmHg, EOA of 2.48 cm2, and RF of 13.75%.
Conclusions:
Data show clinically acceptable hydrodynamic performance of PSIS valves with comparable pressure gradient, flow rate, and EOA to clinically available mechanical and bioprosthetic valves. The PSIS valve from CorMatrix shows a greater EOA and smaller pressure drop, but a larger regurgitation in the mitral compared to tricuspid conditions. Hence transitioning the PSIS valve from its current tricuspid applications to the mitral appears feasible, with a minor concern in regurgitation, though not clinically remarkable.
Student Name: Mohammad Shaver
Title: Mechanical stretch leads to the caveolin-1-dependent release of mineral forming extracellular vesicles from vascular smooth muscle cells
co-authors: Joshua D.Hutcheson
Advisor: Dr.Hutcheson
Lab Link: https://hutchesonlab.fiu.edu
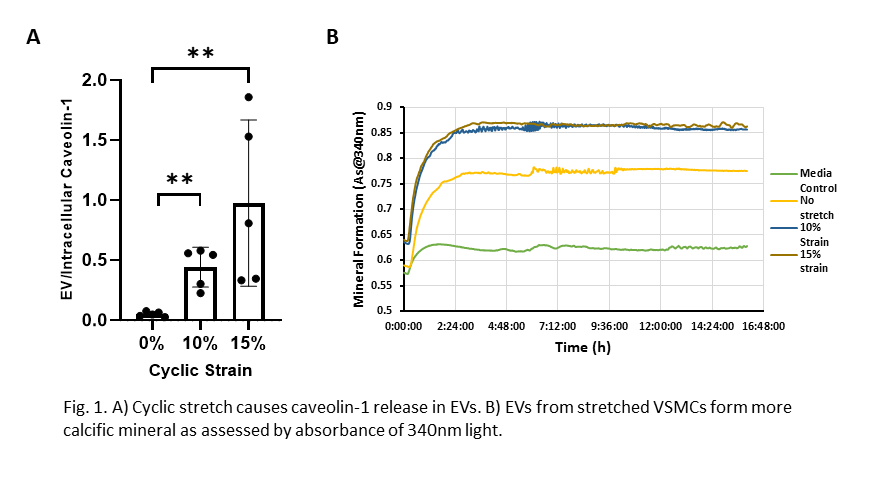
Abstract: Extracellular vesicles (EV), small enclosed structures released from cell membranes, mediate intracellular communication and arterial wall remodeling. Caveolin-1 (Cav-1), an integral structural component of plasma membrane invaginations, is required for the formation of a specific class of EVs released by vascular smooth muscle cells (VSMCs) in pathological vascular remodeling. These EVs nucleate the formation of vascular calcification, which is the most significant predictor of cardiovascular morbidity. Caveolin-1 is a mechanosensitive protein, and VSMCs reside in dynamic cardiovascular tissues. However, the role of mechanics in Cav-1-induced EV formation from VSMCs has not been reported. We hypothesized that pathological levels of mechanical stretch would induce formation of mineral-promoting Cav-1-positive EVs from VSMCs. To test this hypothesis, porcine VSMCs were cultured under two different cyclic stretches (10 and 15%, 0.5Hz) for 72hrs. Compared to non-stretched VSMCs, the 10% and 15% stretch regimes led to 144±4% and 253±14% increases in Cav-1-positive EVs, respectively (Fig.1A). The EVs also appeared bigger than those from non-stretched samples with a 27% and 75% increase in size of EVs from the 10% and 15% stretched VSMCs, respectively. Mineralization potential of EVs can be measured by incubating EVs in phosphate solution and measuring light scattering by mineral at 340nm. The EVs from stretched VSMCs demonstrated higher mineralization potential than the EVs from non-stretched VSMCs (Fig.1B). These data provide new insights into the effect of mechanical stimulation on EV formation and calcifying potential.
Student Name: Anil Thota
Title: Surgical technique enhancements for implantable distributed set of longitudinal intrafascicular electrodes
co-authors: Ranu Jung
Advisor: Ranu Jung
Lab Link: https://ans.fiu.edu
Abstract: Mammalian peripheral neural interfaces (PNI) are used to record, block, and modulate neural signals that relay physiological and pathological signals between the central nervous system and the multitudes of sensory, motor, and autonomic end organs. PNIs can utilize extraneural electrodes or intraneural electrodes that penetrate the epineurium surrounding the nerve. The latter may further penetrate the perineurium surrounding fascicles, which are bundles of nerve fibers within the nerves, for intrafascicular interfaces. We have developed a Neural Enabled Prosthetic Hand system that uses longitudinally inserted intrafascicular fine-wire electrodes (LIFEs) to directly stimulate sensory nerve fibers inside the median and ulnar nerves to provide electrical stimulation for sensory restoration to individuals with upper-limb amputation. Multiple LIFEs are coiled and ensheathed in a silicone tube to protect the wires and provide strain relief. Individual wires emanating from the end of the tube are threaded longitudinally into the fascicle such that the active contact area of the electrode resides inside the fascicle. The length of the wire between the end of the silicone encasing and before insertion into the nerve is surrounded by soft tissue, which may form adhesions to the fragile wires and cause unwarranted stresses. Our goal is to improve the surgical technique to isolate this section of the wires from the surrounding tissue. We propose to develop a surgical technique to physically isolate the unprotected wires from the surrounding tissue. This approach may help reduce unwarranted mechanical stress. Porcine cadaver studies will be conducted to develop the surgical technique. This surgical technique’s feasibility will be tested in an in-vitro sciatic nerve preparation subjected to repetitive linear stress.
