Associate Professor | Interim Chair of Biomedical Engineering
 Research Interests: Biophysical Foundations of Neuroimaging in Normal Aging and Brain Disorders
Research Interests: Biophysical Foundations of Neuroimaging in Normal Aging and Brain DisordersResearch Advancements: Jorge Riera is making strides in treating multiple brain disorders.
Research Area: Therapeutic and Reparative Neurotechnology
Lab: Neuronal Mass Dynamics Laboratory
Website: https://bme.fiu.edu/people/faculty-instructors/jorge-riera/
Biography
Dr. Jorge Riera obtained a B.S. in Physics at the University of Havana in 1988. During 1995-1998, he was “Junior Associate” of the International Centre for Theoretical Physics, Trieste (Italy), where he completed the required credits for a master degree in biophysics. In 1999, he received the Ph.D. degree in Physics from the University of Havana. Part of his Ph.D. thesis was completed at the Pitie-Salpetriere Hospital in Paris. Dr. Riera’s postdoctoral term were first at the RIKEN Brain Science Institute (Japan) and alter at NICHe, Tohoku University (Japan). In 2004, he was appointed associate professor in Tohoku University School of Medicine. From 2006-2011, his research was funded by three Japanese agencies: Japan Society for the Promotion of Science, Telecommunications Advancement Organization of Japan and Japan Science and Technology. In 2011, he joined Florida International University (FIU), first as Visiting Professor and later as Associate Professor in the Department of Biomedical Engineering. For the past ~7 years he has directed the Neuronal Mass Dynamics (NMD) lab. He has also been appointed by the Honor College, the Herbert Wertheim College of Medicine and the STEM Transformation Institute.
Lab Website
https://nmd.fiu.edu/
Research
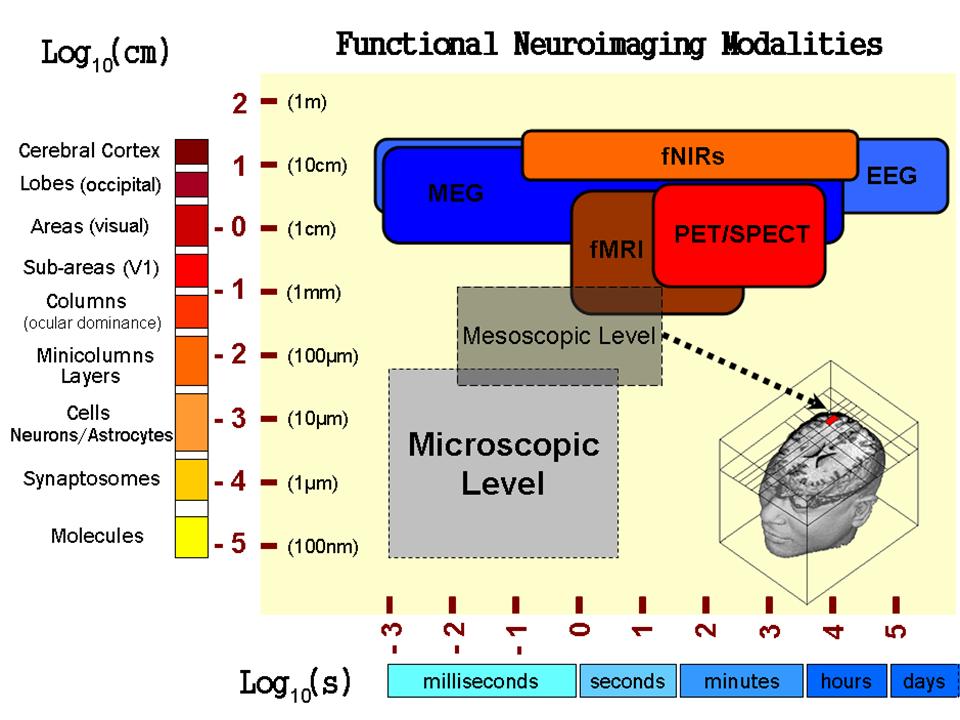
Brain imaging constitutes a powerful tool to study brain functions in both healthy individuals and patients with a variety of brain disorders. The temporal resolution achieved at the present by different modalities allows us to study brain processes occurring from milliseconds to even days. Although much progress has been made, the biophysical foundations of brain imaging are still elusive. In order to identify the fundamental mechanisms underlying the genesis of brain imaging data from a microscopic approach, an intermediate physical level, the mesoscale, needs to be properly described and modeled (Fig. 1). Interpreting a variety of neuroimaging modalities (e.g. EEG/MEG, fMRI, PET/SPECT) at the mesoscopic level from a fundamental neuroscience perspective has been one of the long-term scientific goals in Dr. Riera’s career. In three recent reviews (J Integrative Neuroscience, 5(2), 273-326, 2006; NeuroImage 40, 1436-1459, 2008; Current Opinion in Neurology, 23(4), 374-381, 2010), he has discussed the physiological, biochemical and physical working principles at the mesoscopic level in the cerebral cortex with the strongest impact on the genesis of brain imaging.
At FIU, Dr. Riera performs invasive experiments on rodents, as well as non-invasive studies in humans. The latter are in collaboration with other researchers inside/outside FIU. The animal experiments carried out in Dr. Riera’s group are based on the combinations of pharmacological manipulations (i.e. agonist/antagonist agents) and four main recording techniques (i.e. intracranial electrical potentials obtained with microelectrode arrays, large-scale EEG data, laser Doppler flowmetry and O2/NO amperometric measurements). Through pharmacological manipulations, his group aims to isolate crucial pathways within the neurovascular coupling route and to enhance the activity of particular cortical microcircuit networks both in normal rodents and in those used to model pathological situations. Of particular interest are dementias, epilepsy, hypertension and the cerebro-vascular trauma.
Selected Publications
- Valdés-Hernández PA, Bernal B, Moshkforoush A, Dunoyer C, Khoo HM, Bosch-Bayard J, von-Ellenrieder N, Gotman J, Riera JJ. Identification of negative BOLD responses using windkessel models. bioRxiv Aug. 16, 2018. doi: http://dx.doi.org/10.1101/392290
- Arash Moshkforoush, Pedro A. Valdes-Hernandez, Daniel E. Rivera, Yoichiro Mori, Jorge Riera. waveCSD: A method for estimating transmembrane currents originated from propagating neuronal activity in the neocortex: Application to study cortical spreading depression. J Neuroscience Methods, 307:106-124, 2018.
- Abhay Deshmukh, Jared Leichner, Jihye Bae, Yinchen Song, Pedro A. Valdés-Hernández, Wei-Chiang Lin, Jorge J. Riera. Histological Characterization of the Irritative Zones in Focal Cortical Dysplasia Using a Preclinical Rat Model. Frontiers in Cellular Neuroscience 12(52): 1-15, 2018.
- Bruyns-Haylett M, Luo J, Kennerley AJ, Harris S, Boorman L, Milne E, Vautrelle N, Hayashi Y, Whalley BJ, Jones M, Berwick J, Riera J, Zheng Y. The neurogenesis of P1 and N1: A concurrent EEG/LFP study. NeuroImage 146, 575-588, 2017. IF: 5.835
- Song Y, Garcia S, Frometa Y, Ramella-Roman JC, Soltani M, Almadi M, Riera J, Lin W-C. Quantitative assessment of hemodynamic and structural characteristics of in vivo brain tissue using total diffuse reflectance spectrum measured in a non-contact fashion. Biomedical Optics Express 8(1), 78-103, 2017.
- Valdes-Hernandez PA, Bae J, Song Y, Sumiyoshi A, Aubert-Vazquez E, Riera J. Validating Non-invasive EEG source imaging using optimal electrode configurations on a representative rat head model. Brain Topography 29, 1-26, DOI 10.1007/s10548-016-0484-4, 2016. (Corresponding author).
- Song Y, Torres RA, Garcia S, Frometa Y, Bae J, Deshmukh A, Lin W-C, Zheng Y, Riera J. Dysfunction of neurovascular/metabolic coupling in chronic focal epilepsy. IEEE Trans. Biomed. Eng. 63(1), 97-110, 2016. (Corresponding author). IF: 3.577
- Song Y, Riera J, Bhatia S, Ragheb J, Garcia C, Weil AG, Jayakar P, Lin W-C. Intraoperative optical mapping of epileptogenic cortices during non-ictal periods in pediatric patients. NeuroImage: Clinical 11, 423-434, 2016.
- Saito A, Mekawy MM, Sumiyoshi A, Riera J, Shimizu H, Kawashima R, Tominaga T. Noninvasive targeting delivery and in vivo magnetic resonance tracking method for live apoptotic cells in cerebral ischemia with functional Fe2O3 magnetic nanoparticles. J Nanobiotechnol 14(19), 1-11, 2016.
- Song Y, Sanganahalli BG, Hyder F, Lin WC, Riera J. Distributions of irritative zones are related to individual alterations of resting-state networks in focal epilepsy. PLoS ONE 10(7): e0134352. doi:10.1371/journal.pone.0134352, 2015.
- Bae J, Deshmukh A, Song Y, Riera J. Brain source imaging in rats with preclinical models of focal epilepsy using high-resolution EEG recordings. J Vis Exp (100), e52700, doi:10.3791/52700, 2015.
- Riera J, Goto T, Kawashima R. An electrophysiological microscope for fast assessments to the activity of cortical networks in vivo: from population inputs to single unit outputs. Frontiers in Neural Circuits 8(4), 1-14, 2014.
- Riera J, Cabo A. Instantaneous charge unbalance in the brain: a result from procedural errors or an authentic physical phenomenon? Journal of Neurophysiology 109, 1684-1685, 2013.
- Wang K, Riera J, Enjieu Kadji H, Goto T, Kawashima R. The role of the extracellular impedance profiles in the compartmental models for neurons: a unified formalism for recording and stimulation. Neural Computation 25, 1807-1852, 2013.
- Aizawa-Kohama M, Endo T, Kitada M, Wakao S, Sumiyoshi A, Matsuse D, Kuroda Y, Riera J, Kawashima R, Tominaga T, Dezawa M. Transplantation of bone marrow-derived neural precursor cells ameliorates deficits in a rat model of complete spinal cord transection. Cell Transplantation 22(9), 1613-1625, 2013.
- Riera J, Ogawa T, Goto T, Sumiyoshi A, Nonaka H, Evans A, Miyakawa H, Kawashima R. Pitfalls in the dipolar model for the neocortical EEG sources. Journal of Neurophysiology 108(4), 956-975, 2012.